Decoding the DRAP SRO 470 Barcoding Project
As of today, SRO 470(i)/2017 is effect vide letter 26th April 2018 issued by the DRAP to all the stakeholders of the industry. SRO 470(i)/2017 dictates that the following information needs to be printed on the labels.
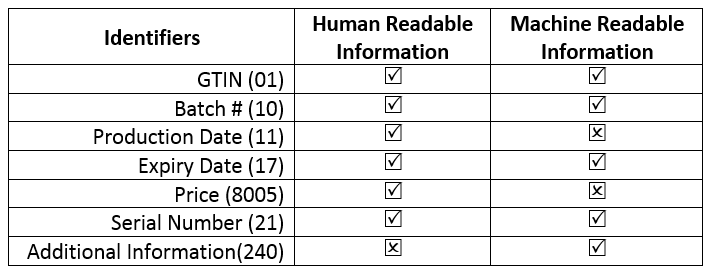
However, a tacit understanding has been reached on part of the regulatory and the industry that serialization is being postponed to be implemented by June 30th 2019 in Pakistan. Do note that no official directive exists saying the Serialization is delayed or recalled. AI240 has also been relieved from its utility.
What does Punjab Government Tender Requires
Punjab Tender primarily requires SRo 470(i)/2017 to be implemented. The Chief Drug Controller Office in Lahore primarily acts as the vigil for implementation of the same. They require GTIN, Batch #, Expiry Date and Serial # embedded in the Barcode as Machine Readable information, along with compliance of Drug Labelling Rules 1986 for Human Readable Information.
At times they require AI 240 or AI 90 to be embedded in the barcode for further security of the product.
As long as these requirements are met, the product is valid and is acceptable to CDC office for circulation at their respective outlets.



